
Contact Us

Transcranial magnetic stimulation (TMS) over the left dorsolateral prefrontal cortex (L-DLPFC) is an FDA-approved treatment for conditions such as treatment-resistant depression (TRD). Still, it is only partially effective, with response and remission rates of 41.2% and 35.3%, respectively. The FDA-approved protocol for TRD identifies the left DLPFC stimulation site by moving the coil 5 cm anterior to the “hand motor hotspot” (motor cortex) along the curvature of the scalp—a one-size-fits-all approach. This method provides only approximate targeting of the left DLPFC, often missing the DLPFC entirely, with no consistent differentiation among potentially relevant DLPFC subregions.
One reason some people respond well while others do not may be individual variations in brain organization, which would require stimulation to be applied to slightly different locations for different individuals.
An important goal of TMS therapy is to guide TMS targeting on a personalized basis to improve consistency across individuals and enhance outcomes for a range of mental health conditions.

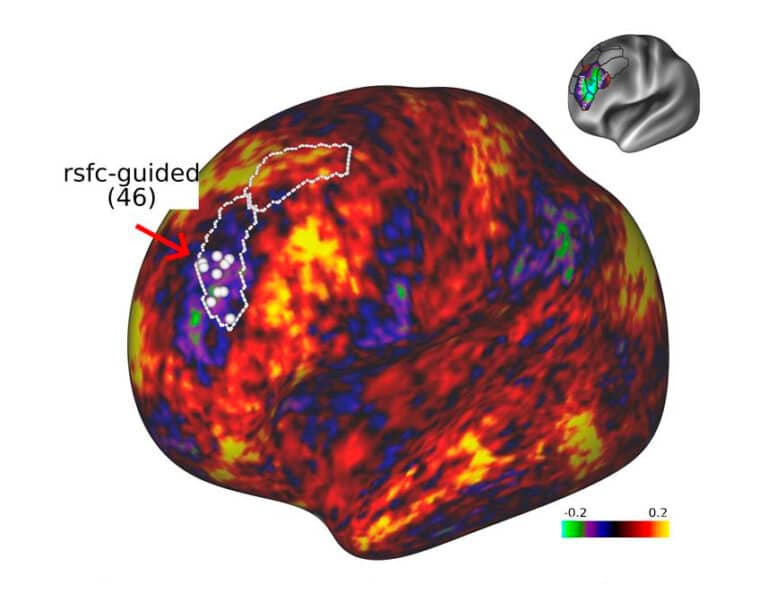
Alternative methods to DLPFC target identification can be used to personalize treatments using intrinsic (resting state) functional connectivity MRI, a powerful imaging technique that applies correlations in spontaneous fluctuations in the blood oxygen level-dependent signal to assess functional relationships between brain regions.
The differences in brain organization can be detected using individualized functional MRI scans, which then allow the coil placement to be determined separately for each individual.
View sources here: https://neurotherapeutixnyc.com/sources/
Call us at (917) 388-3090 or click to request a regular or telehealth appointment.
Neurotherapeutix
171 East 74th Street, Unit 1-1 New York, NY 10021

Neurotherapeutix is the leading clinic for functional imaging guided transcranial magnetic stimulation (TMS), a safe, innovative, and non-invasive methodology for treating a wide range of acute and chronic mental disorders and brain injuries. Our advanced fMRI technology allows us to map the brain for the… Learn More »
By: Neurotherapeutix NYC
Reviewed By: Marta Moreno, Ph.D
Published: March 24, 2023
Last Reviewed: September 27, 2024
QUICK INQUIRY
Contact us to get an estimate for your medical services requirements. You can fill in the form to specify your medical requirements or you can call us directly.